Center for Tobacco Products (CTP) Map
Center for Tobacco Products (CTP) Map
Knowledge•Action•Change (2018)- No Fire, No Smoke: The Global State of Tobacco Harm Reduction
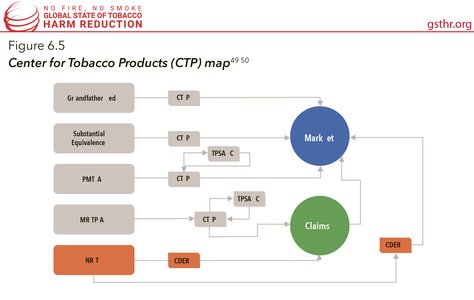

The FDA in the USA has long held the authority to regulate nicotine replacement therapies such as gum, patch, and lozenges through the Center for Drug Evaluation and Research (CDER). In 2009, with the passage of the Family Prevention Smoking and Tobacco Control Act, the FDA gained the authority to regulate tobacco products and marketing for cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco. On May 10, 2016, the FDA published a final ‘deeming rule’ allowing the agency to begin regulating all tobacco products, including cigars, pipe tobacco, waterpipes (or hookahs), dissolvable products, e-cigarettes, and other electronic nicotine delivery systems.
The deeming rule requires that any new product (any tobacco product not commercially marketed in the US as of February 15, 2007) must go through a Pre-Market Tobacco Application (PMTA) process to stay on the market (the deadline to submit a PMTA was August 8, 2022 for non-combustible products and August 8, 2021 for combustible products), and a Modified Risk Tobacco Product Application (MRTPA) in order to make any health claims about relative risk compared to continuing to smoke cigarettes. Products that have been subjected to small changes from the original “grandfathered product” (products on the market before February 15, 2007) must submit a Substantial Equivalence Report (SE) or an Abbreviated SE report so the product can stay on the market without a PMTA. This infographic maps the pathways a nicotine containing product can take to get to market). The PMTA pathway requires that the applicant proves that the product would be appropriate for the protection of the public health. The statute does not define that standard further. The FDA has released draft guidance documents, however, at the time of writing, none of them have been finalised meaning that they are not enforceable and the agency can change them at any time. To date, only eight PMTA orders have been granted for Swedish Match’s General Snus products.
See also p. 93 of the report: No Fire, No Smoke: The Global State of Tobacco Harm Reduction 2018 — Global State of Tobacco Harm Reduction (gsthr.org)